Artistic representation of an atom. Image generated using Ideogram AI.
By Mariana Meneses
What binds us together?
Depending on your area of expertise, you may have different answers for this. Social scientists may say, for example, that social networks rooted in our most inherent desire for connection and belonging are the main binding force reinforcing collaboration through pro-social behavior; while evolutionary scientists may cite biological mechanisms such as sharing similar physical features and genomes. For chemists and physicists, however, the readiest answer may relate to the nuclei of atoms.
Consisting of protons and neutrons bound together by the strong nuclear force, the nucleus represents a delicate balance of forces within the atom. The nuclei of atoms lie at the heart of understanding interactions of fundamental particles, but recent experiments with helium-4 nuclei have unveiled perplexing discrepancies between theoretical predictions and observed behaviors, challenging our current understanding of nuclear forces.
Helium-4 is the most common isotope of helium, the chemical element that powers stars like our sun, with a nucleus consisting of two positively charged protons and two neutrons having no charge.
Strong nuclear force refers to the fundamental interaction between protons and neutrons within atomic nuclei, responsible for binding them together despite the electrostatic repulsion between positively charged protons. This force is mediated by exchange particles called mesons, particularly pions (subatomic particles composed of a quark and an antiquark) and is characterized by its short-range nature and its ability to overcome the electromagnetic force, allowing for the stability of atomic nuclei.
While theories like chiral effective field theory have provided significant insights into nuclear interactions, they seem insufficient to explain the complexities observed in the helium nuclei.
This discrepancy complicates the ongoing quest to refine our understanding of nuclear forces, crucial for unraveling the mysteries of atomic nuclei and their role in shaping the universe’s fabric.
Chiral Effective Field Theory is a tool used by scientists to understand how the tiny particles inside an atom’s nucleus interact with each other. It’s like a rulebook that helps us understand which forces are most important when these particles are not moving very fast. The theory works well with computer simulations that represent these particles and their interaction, however, despite its utility, ongoing research aims to refine and expand the theory to tackle unresolved questions and enhance our understanding of nuclear forces. (Cornell University).
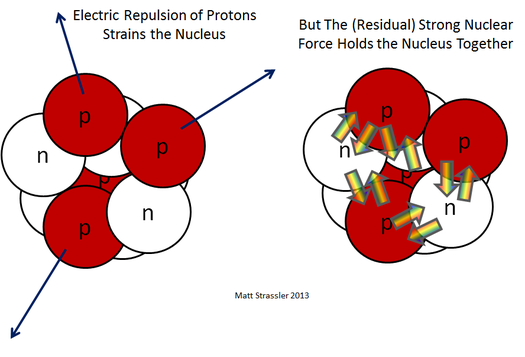
“The electrical force pushing protons apart and the strong force acting on both protons and neutrons inside of a nucleus.” Credit: Prof. Matt Strassler
The atom, as understood through centuries of scientific inquiry and discovery, is the cornerstone of modern chemistry and physics.
Initially conceptualized by ancient Greek philosophers such as Democritus as an indivisible particle, the atom’s definition underwent transformative milestones. John Dalton’s atomic theory in the 19th century laid the groundwork, depicting atoms as uniform, hard spheres. Subsequent revelations by JJ Thomson, showcasing the existence of subatomic particles like electrons, and Ernest Rutherford’s gold foil experiment, revealing the atomic nucleus composed of protons and neutrons, reshaped atomic understanding.
Niels Bohr’s planetary model further refined the atomic structure, proposing electron orbits in discrete energy levels around the nucleus. The advent of quantum mechanics in the 20th century brought a paradigm shift, introducing probabilistic wave functions and quantized energy levels, revolutionizing our comprehension of the atom’s behavior.
In new research using helium-4 nuclei, by Dr. Simon Kegel and colleagues from the Johannes Gutenberg-Universität Mainz, physicists aimed to measure the strong nuclear force by observing the expansion of helium nuclei when excited, expecting theoretical predictions to align with experimental results. However, the observed expansion exceeded expectations of current theories, notably the chiral effective field theory.
The unexpected findings suggest that crucial components of the nuclear force, sometimes overlooked, might play a more significant role than previously assumed, potentially necessitating a reassessment of fundamental principles in nuclear physics. Further investigations are underway to test this discrepancy, which could potentially reshape our understanding of the forces governing atomic nuclei.
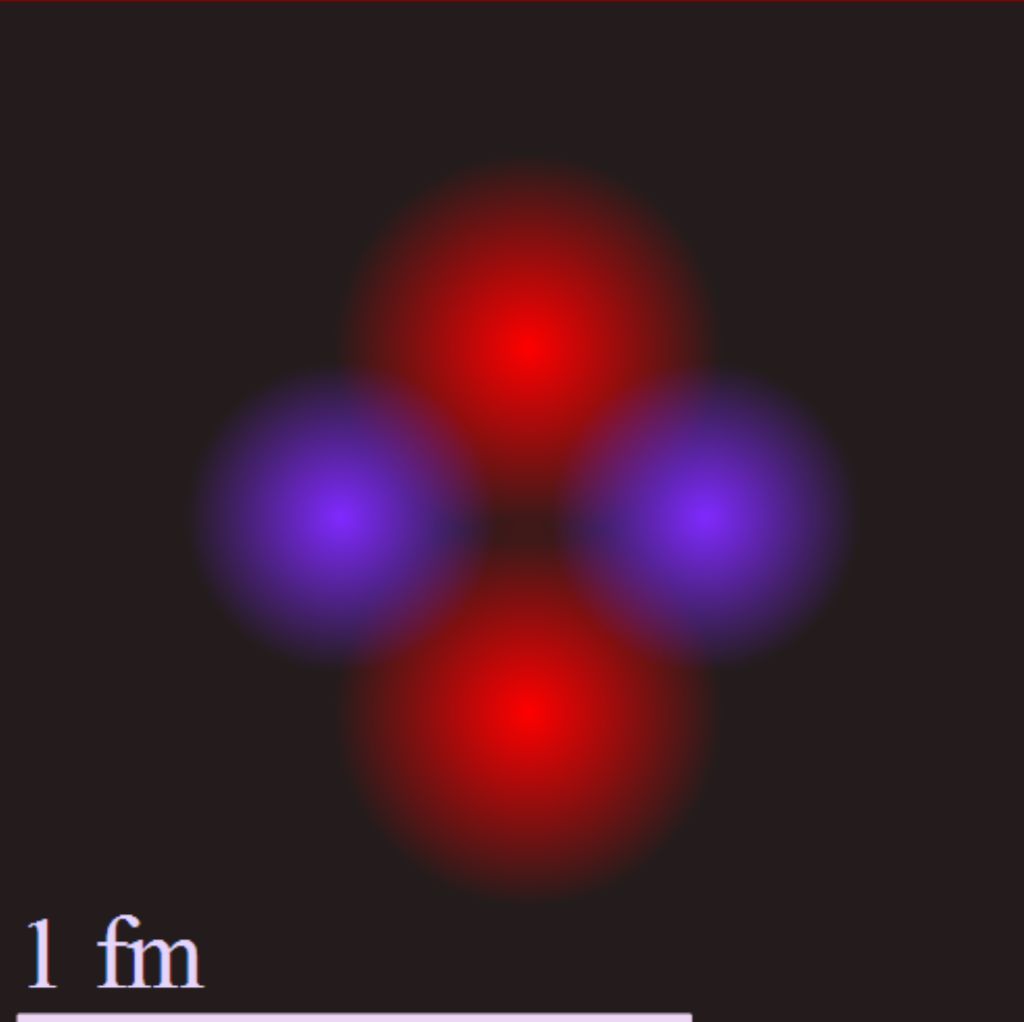
The diagram shows a helium atom’s nucleus with protons in pink and neutrons in purple, at a scale of 1 femtometer (fm), which is 1/1,000,000,000,000,000th of a meter. Credit: Yzmo
Another breakthrough in the matter of matter comes from an international research team at Michigan State University’s Facility for Rare Isotope Beams (FRIB).
In collaboration with the Institute for Basic Science in South Korea and RIKEN, in Japan, the researchers have successfully created five new isotopes — thulium-182, thulium-183, ytterbium-186, ytterbium-187, and lutetium-190—marking a significant milestone in nuclear physics.
These isotopes, reported in Physical Review Letters, are the first of their kind produced at FRIB and provide insights into nuclear processes occurring in ultra-dense celestial bodies like neutron stars. The achievement demonstrates FRIB’s capability to replicate conditions like stellar environments, facilitating experiments previously impossible on Earth. This breakthrough not only provides crucial insights into how heavy elements, like gold, are formed but also opens doors for further research in understanding the fundamental properties of atomic nuclei.
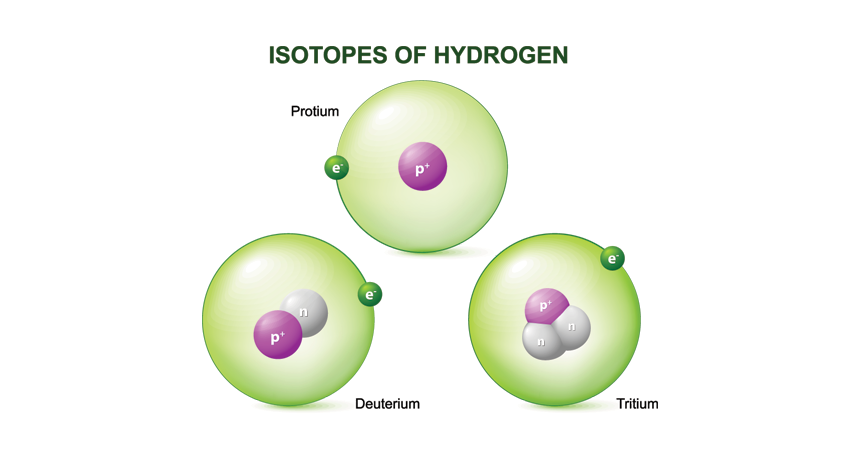
“This image shows three isotopes of hydrogen. Each has one proton (purple) but a different number of neutrons (grey).” (Science News) Credit: Roxana Balint
An isotope is a variant of a chemical element that has the same number of protons (and hence the same atomic number) but differs in the number of neutrons in its nucleus. This difference in neutron number results in a different atomic mass, but it does not affect the chemical properties of the element. This is because chemical properties are determined by the number of electrons, which equals the number of protons in a neutral atom.
Ongoing research on different aspects of atomic nuclei, ranging from the exploration of nuclear forces and isotopes to the creation of new variants of known chemical elements, enriches our understanding of the fundamental properties of matter, from its microscopic constituents to its macroscopic behaviors.
In another exciting development, scientists have recently identified a hidden phase transition between liquid and solid states of matter, providing another significant advancement in our comprehension of the intricate properties and behaviors of matter at both the atomic and molecular levels.
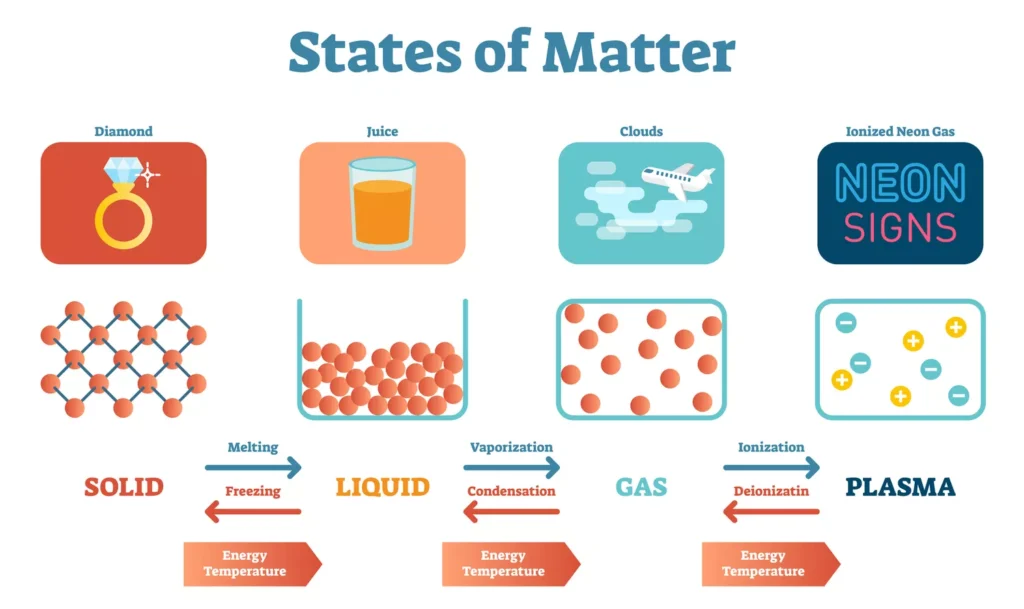
“A state of matter is defined as one of the ways in which matter can interact with itself to form a homogeneous phase.” (Anne Marie Helmenstine)
Onset temperature
Researchers at Lawrence Berkeley National Laboratory, led by Dimitrios Fraggedakis, have uncovered a hidden phase transition in amorphous materials like plastic and glass, shedding light on the molecular behavior behind their transition from a liquid-like to a solid-like state at a certain temperature known as the onset temperature.
Unlike crystalline solids, amorphous materials do not form orderly structures upon cooling but instead remain in a supercooled liquid state. Through a combination of theory, computer simulations, and experiments, the scientists demonstrated that the molecules in these materials become extremely viscous as they approach the onset temperature, barely moving and exhibiting solid-like behavior. This newfound understanding could lead to the development of novel amorphous materials for various applications, including medical devices and drug delivery.
Onset temperature refers to the pivotal temperature at which a reaction or phase change initiates, such as melting or crystallization, signifying the point at which the system’s behaviour shifts distinctly.
“Artist’s illustration of an ‘isolated neutron star’ — one without associated supernova remnants, binary companions or radio pulsations.” (DESCRIPTIONPen State University)
The progress of scientific inquiry into the fundamental properties of matter.
The recent strides in atomic and molecular research, from shedding new light on nuclear forces and generating new isotopes to uncovering hidden phase transitions in amorphous materials, underscore the remarkable progress of scientific inquiry into the fundamental properties of matter. These breakthroughs not only deepen our understanding of the behaviors of atoms and molecules but also hold immense promise for practical applications in various fields, from advancing our comprehension of stellar phenomena to fostering the development of innovative materials for medical and technological purposes.
As scientists continue to delve into the mysteries of the universe, these discoveries illuminate the awe-inspiring journey of exploration and the boundless potential of human curiosity.
Craving more information? Check out these recommended TQR articles:
- Shedding a New Light on Electrons: 2023 Nobel Prize in Physics Awarded for Imaging Fundamental Particle
- Magnetic Mysteries Beyond Our Solar System Could Help Us Find Life Out There
- AI Advances the Use of Stem Cells in Personalized Medical Treatment
- Life Beyond Plastic: How Insects and Enzymes Could Hold the Key to Solving Plastic Pollution
- Water’s Cosmic Origins, and its Role in Earth’s Rotational Balance, Amplify the Need for Responsible Use
- Increasing Precision in Time Measurement, From the Atom to the Cosmos
- Finding Truths: Defense and Criticism of the Scientific Method