
Vu-Thi Thu-Thao, centre, was freed from the debilitating symptoms of lupus, an autoimmune disease, in early 2021 with the help of CART-T technology. Image: S. Kind/University Hospital Erlangen, reported in Süddeutsch Zeitung .
By James Myers
The immune system is a remarkable feature of human biology, and recent scientific discoveries are revealing much more about the system’s defence mechanisms that keep us safe from a wide array of tiny and dangerous pathogens. The discoveries continue to advance life-saving cancer treatments and are also relieving the debilitating symptoms of some autoimmune diseases, which occur when the body’s defences malfunction and attack its own tissues.
Millions of people around the globe suffer from autoimmune diseases like lupus and multiple sclerosis, and relief is in sight with treatments using CAR-T technology.
In early 2021, CAR-T (which is short for Chimeric Antigen Receptor, T-cell) was used to treat 20-year-old Vu-Thi Thu-Thao. Thu-Thao was a lupus victim suffering from the joint pain and breathing difficulties common to the disease, who four years later is still free of symptoms and no longer requires a daily regimen of up to 20 pills.
Chimeric Antigen Receptors are proteins that have been engineered to give the body’s own T cells, which are a type of white blood cell that forms the front line of the immune system’s defence against invaders, the ability to recognize and attack specific pathogens that cause disease.
Thu-Thao’s autoimmune treatment had been adapted from one that is applied to some cancer patients. She underwent three days of chemotherapy to kill her immune cells and make way for lab-engineered replacements. The replacement cells had been manufactured using the same process that targets other types of blood cells, called B cells, which manufacture invader-fighting antibodies. B cells can become cancerous and can also trigger autoimmune diseases, so the B cell manufacturing process proved effective for T cells too.
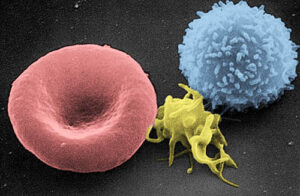
Scanning electron micrograph of a red blood cell (left), a platelet (center), and a T lymphocyte (right). A T cell is a form of lymphocyte. Colorized image by Electron Microscopy Facility at The National Cancer Institute on Wikipedia.
Thu-Thao’s CAR-T treatment was tailored specifically to her T cells.
T cells originate as stem cells in bone marrow, and they mature in the thymus, which is a principal organ of the immune system that’s located in the upper chest. The different varieties of T cells include CD8+ “killer T cells” that directly kill virus-infected cells in the body, CD4+ “helper T cells” that trigger a wider response from the immune system, and regulatory “suppressor” T cells that distinguish between invading cells and the body’s natural immune cells.
Malfunction of the regulatory T cells is a cause of autoimmune diseases like lupus and a major factor in the spread of cancer. Cancer cells can multiply by seizing control of regulatory T cells, so they can masquerade as healthy cells and prevent an immune system response. Significant advancements in treating cancer have been achieved with technologies like CAR-T that adjust the immune system, boosting its ability to detect and fight invasions of cancerous cells.
Immune therapy has been particularly successful in treating melanoma.
Melanoma is the most dangerous form of skin cancer that is typically triggered by overexposure to sunlight, sometimes in combination with a weakened immune system, that damages the DNA of skin cells. While localized melanoma cells can often be treated successfully, new immune therapies are giving hope to patients with aggressive melanoma that has spread through the body’s lymph nodes.
Lymph nodes are a primary home for the immune system’s T cells and act to filter foreign particles and pathogens from the body. Lymph nodes are also the major site of the antibody-manufacturing B cells, which are, like T cells, white blood lymphocytes. The role of the lymphocytes is to secrete antibodies and other molecules that attach to foreign particles and allergens and provoke an immune system response.
Melanoma cells can hijack a molecule called PARP14 to suppress the immune system, but earlier this year Dr. Adam Hurlstone and his team at the University of Manchester identified genetic signals in melanoma cells that indicate where and when PARP14 molecules can be successfully blocked. The results of their work were published in the Journal for ImmunoTherapy of Cancer in January.
Moving forward, more research will be able to add to our understanding how blocking PARP14 molecules could combine with other technological treatments to supercharge the immune system’s power for fighting melanoma and other deadly cancers.
Dr. Hurlstone is investigating the potential combination of a PARP14 blocking treatment with drugs called immune checkpoint inhibitors. Immune checkpoint inhibitors are being used to treat many other types of cancer, including breast cancer, cervical cancer, colon cancer, and lung cancer.
Immune checkpoints are a normal feature of a healthy immune system. They act like brakes, preventing the immune response from becoming so strong that it damages the body’s own cells. They are activated when the proteins on the surface of T cells bind to specific partner proteins on other cells. Some cancer cells exploit this system by mimicking those partner proteins, tricking the checkpoints into turning off the T cells. Drugs known as immune checkpoint inhibitors stop the proteins in the checkpoints from combining with partner proteins, allowing the immune response to continue fighting.
What’s in store for immune therapies of the future?
The rapid pace of scientific discoveries makes it difficult to predict the extent to which we will be able to engineer our immune systems for life-saving treatments. However, adopting preventive measures is also a key approach to avoiding diseases and autoimmune conditions before they inflict damage on the body. A new test has been developed to measure the health of an individual’s immunome, which could allow for early intervention and repair before symptoms appear.
The human immunome is a term applied to the 1.8 trillion cells that inhabit our bodies, together with trillions of proteins, metabolites, mRNA, and other biomolecules that comprise the vast network monitored by our immune systems. Each person’s immunome differs because of genetics, past illnesses, diet, physical fitness, environment, and a host of other factors.
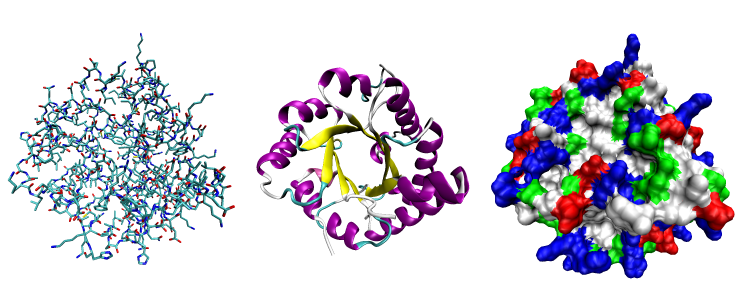
Consisting of one or more chains of biomolecules, proteins are key elements in the operation of our immune systems. Depicted above are different three-dimensional representations of the protein triose phosphate isomerase: the image on the left shows the protein’s various atoms distinguished by colour, the middle image illustrates the protein’s chain framework, and the image on the right uses different colours to indicate the locations and types of solvents to which the protein is susceptible. Images by Opabinia regalis – Self-created from PDB entry 1TIM using the freely available visualization and analysis package VMD, on Wikipedia.
A study entitled A unified metric of human immune health, by Rachel Sparks, John Tsang, and colleagues, published in Nature Medicine in 2024, points to similarities in immune system disruptions among patients with different diseases. The authors used machine learning on clinical data from 228 patients with 22 different conditions and compared them to a control group of 42 healthy people. The goal was to correlate symptoms and generate a score, or metric, that identifies individuals whose immune systems are at particular risk.
The technology behind the new immune system metric can assess up to one million cells, proteins, mRNA, and immune biomolecules. It’s one of several technologies that are enabling precision and individualized medical treatment for a specific patient’s conditions, optimized to target and treat unhealthy cells while preserving the healthy cells. The new metric could also advance understanding of why individuals react differently to a virus like Covid-19 and might one day become part of routine physical examinations to help with disease prevention.
Moreover, while some researchers focus on specific aspects of the immune system, others are investigating its operation holistically. Founded in 2007, the Stanford University Human Immune Monitoring Center is one example of a unified approach to the human immune system that, combined with discoveries on its particular mechanisms, holds promise for revolutionizing the treatment of autoimmune conditions, cancer, and other diseases.
There’s a saying that “sometimes the best defence is a good offence.” Our immune systems already stand guard on the front lines against countless threats, and now science is enhancing their power, helping them not just to defend but to seek out and destroy the invaders.
Craving more information? Check out these recommended TQR articles:
- Thinking in the Age of Machines: Global IQ Decline and the Rise of AI-Assisted Thinking
- Everything Has a Beginning and End, Right? Physicist Says No, With Profound Consequences for Measuring Quantum Interactions
- Cleaning the Mirror: Increasing Concerns Over Data Quality, Distortion, and Decision-Making
- Not a Straight Line: What Ancient DNA Is Teaching Us About Migration, Contact, and Being Human
- Digital Sovereignty: Cutting Dependence on Dominant Tech Companies





