What if the future of technology is less about replacing biology, and more about tuning it? Image: Gerd Altmann, on Pixabay.
By Mariana Meneses
For decades, advanced technology has approached biology as something to be fixed, overridden, or replaced. But a new wave of research is quietly redefining that relationship. Recent studies across medicine, aging, and even quantum physics reveal a shared insight: some of the most powerful interventions do not introduce entirely new systems, but instead amplify, restore, or uncover capacities already embedded in living matter.
In a peer-reviewed scientific paper published in October 2025 in the journal PNAS, titled “Nanomaterial-induced mitochondrial biogenesis enhances intercellular mitochondrial transfer efficiency”, researchers led by John Soukar, from the Department of Biomedical Engineering at Texas A&M University, set out to enhance a natural but inefficient repair process in the body: the transfer of mitochondria between cells. Mitochondria are often described as the “power plants” of cells because they produce most of the energy that cells need to function. When mitochondria are damaged, as happens in many diseases, including heart, muscle, and neurodegenerative disorders, cells struggle to survive. Although some cells, especially stem cells, can donate healthy mitochondria to damaged neighbors, this transfer usually happens at low rates, limiting its therapeutic potential.
To address this problem, the researchers used a specially engineered nanomaterial, a chemical compound of molybdenum disulfide (MoS₂) at an extremely small scale, shaped like tiny “nanoflowers.” These nanoflowers were designed with atomic-scale defects that allow them to interact with the cell’s internal chemistry. When human mesenchymal stem cells were exposed to these nano-materials, the cells began producing many more mitochondria than usual—in fact, about twice as many! In effect, the stem cells were turned into “mitochondrial bio-factories.” Importantly, this boost did not harm the cells. Instead, it increased their energy production and overall metabolic capacity, making them better suited to help other cells in need.
“Mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells or medicinal signalling cells, are [specific types of] cells that can differentiate into a variety of cell types, including osteoblasts (bone cells), chondrocytes (cartilage cells), myocytes (muscle cells) and adipocytes (fat cells which give rise to marrow adipose tissue). The primary function of MSCs is to respond to injury and infection by secreting and recruiting a range of biological factors, as well as modulating inflammatory processes to facilitate tissue repair and regeneration” (Wikipedia).
The increase in mitochondrial numbers had a direct effect on how efficiently mitochondria were shared between cells. Using live imaging and molecular tracking, the researchers showed that stem cells treated with MoS₂ nanoflowers transferred mitochondria to neighboring cells at much higher rates that were sometimes three to four times higher than untreated cells. This transfer occurred through physical connections between cells called tunneling nanotubes, which act like tiny bridges. The key point is that the nanomaterial did not force cells to behave unnaturally; rather, it amplified a process that already exists by giving donor cells more mitochondria to share.
Finally, the team tested whether this enhanced transfer helped damaged cells recover. In several models where cells were deliberately injured, such as by drugs that disrupt mitochondrial function, the transferred mitochondria restored energy production, reduced harmful oxidative stress, and improved cell survival. In heart-related cells damaged by the chemotherapy drug doxorubicin, a well-known cause of cardiac toxicity, mitochondrial transfer from nanomaterial-treated stem cells significantly reversed cellular damage. While these results are still limited to laboratory experiments, the study provides clear proof of concept: by using nanomaterials to strengthen the body’s own repair mechanisms, it may be possible to develop new treatments for diseases rooted in mitochondrial dysfunction.
In sum, this study shows that carefully designed nanomaterials can be used to amplify the body’s own capacity for repair. By turning stem cells into more efficient mitochondrial donors, the researchers demonstrate a new strategy for addressing diseases linked to energy failure at the cellular level. Rather than targeting symptoms or delivering external replacements, this approach works by strengthening natural cooperation between cells. If future studies confirm its safety and effectiveness in living organisms, nanomaterial-enhanced mitochondrial transfer could open a new direction in regenerative medicine.
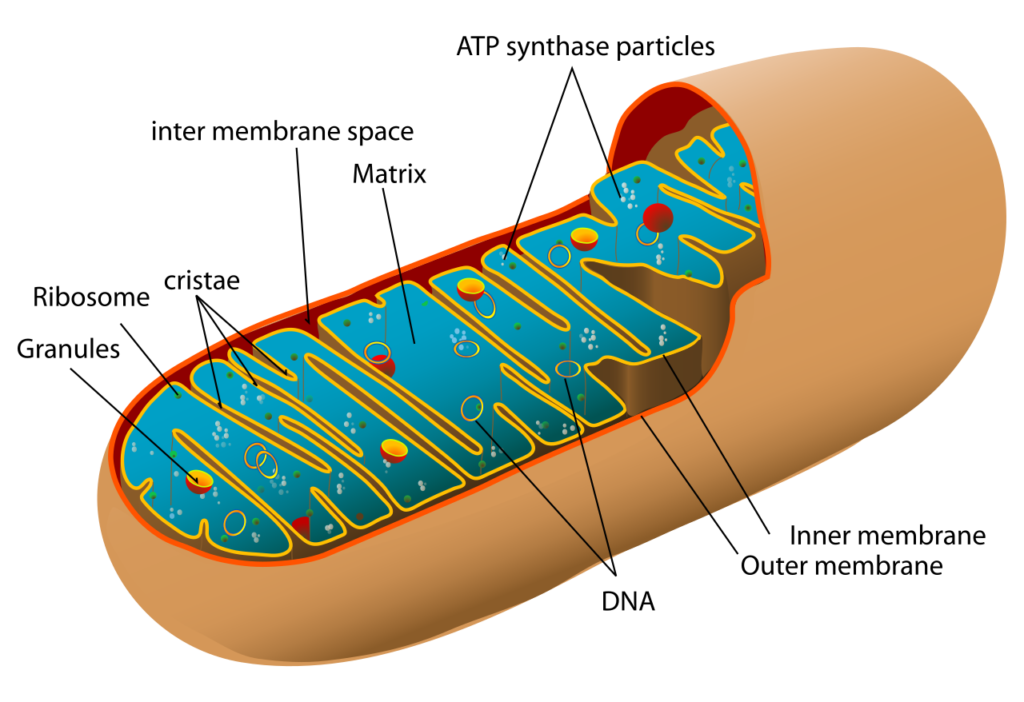
“The diagram shows a cross-section of a eukaryotic cell mitochondrion.” Image: Mariana Ruiz Villarreal LadyofHats, Wikipedia .
If the mitochondrial study shows how technology can enhance cooperation between individual cells, a second line of research extends this logic to an entirely different biological scale: the coordination of whole organs. In a study published in December 2025 in the journal Nature, titled “Transient hepatic reconstitution of trophic factors enhances aged immunity”, researchers led by Mirco J. Friedrich, from the Massachusetts Institute of Technology, investigated a central problem of ageing: the gradual weakening of the immune system.
As people age, the body produces fewer new immune cells—especially T cells, which are critical for fighting infections, responding to vaccines, and controlling cancer. Last November, in “Immune Therapies Advance Rapidly from Disease Control to Possible Cure for Cancer”, The Quantum Record examined the central role of T cells in immune health, highlighting how recent therapies such as CAR-T work by enhancing the body’s own immune defenses rather than replacing them. These advances illustrated a broader shift in medicine: from suppressing or rebuilding the immune system to selectively strengthening the conditions under which key immune cells can function effectively
The new study notes that previous attempts to rejuvenate immunity have had limited success or serious side effects and thus explores a new strategy: temporarily repurposing the liver to restore key immune signals (like the messages that cells exchange to coordinate the function and achieve the goal) that decline with age, rather than trying to directly repair ageing immune organs themselves.
To identify which immune signals were missing in old age, the team first conducted large-scale molecular mapping of immune tissues in young and aged mice. They found that several “trophic factors,” which are molecules that support immune cell development and survival, decline sharply with age—in particular, reduced molecules that normally help generate new T cells and maintain a diverse and responsive immune system. Their decline helps explain why older individuals struggle to respond to new infections or vaccines.
Rather than delivering these molecules directly as drugs, which often requires high doses and can trigger inflammation, the researchers used messenger RNA (mRNA) technology, which proved highly effective in the rapid development of Covid-19 vaccines. They packaged mRNAs and injected them into aged mice, selectively delivering the mRNA to liver cells, which then temporarily produced and released the immune-supporting factors into the bloodstream. The liver was chosen because it remains metabolically active even in old age and naturally interacts with circulating immune cells. Importantly, the effects were transient: once the treatment stopped, protein production faded without permanently altering the tissue.
This temporary immune “boost” had striking effects. Aged mice treated with the mRNA combination produced more new, naïve T cells, showed improved responses to vaccination, and mounted stronger anti-tumor immune responses. In cancer models, treated mice had greater activation of tumor-specific immune cells and responded better to immunotherapy. Crucially, unlike traditional treatments, this approach did not trigger harmful inflammation or autoimmune reactions. It demonstrates how the immune system became more effective without becoming overactive, a balance that has been difficult to achieve in previous anti-ageing immune interventions.
In sum, this study demonstrates that ageing-related immune decline may be reversible, at least in part, by restoring the signals that immune cells depend on, rather than replacing the cells themselves. By using mRNA to turn the liver into a temporary “factory” for immune-supporting factors, the researchers show a new way to strengthen immunity safely and reversibly. While these results are so far limited to animal models, they point to a promising direction for future therapies aimed at improving vaccine responses, cancer immunity, and overall immune resilience in ageing populations.

What benefits do you see in anti-ageing research? Image: Gerd Altmann, on Pixabay .
At the most fundamental level, ageing begins inside individual cells as they gradually lose the ability to divide, repair damage, and maintain stable genetic programs. One of the most ambitious goals of modern biomedicine is therefore not simply to treat age-related diseases, but to slow, or even partially reverse, the cellular processes that drive ageing itself without triggering dangerous side effects.
A study featured in the upcoming March 2026 edition of Genes & Diseases takes on this challenge directly, asking whether it is possible to restore youthful cellular function while avoiding the cancer risks that have long limited reprogramming-based approaches. The study was titled “Optimized Yamanaka factors combined with TERT gene therapy for enhanced anti-aging effects” and led by Mengmeng Jiang, from the Shenzhen University.
Previous work showed that Yamanaka factors, which consist of four genes known as Oct4, Sox2, Klf4, and c-Myc, can partially “reprogram” aged cells and restore youthful features. However, the set (abbreviated OSKM) is dangerous when used continuously, largely because c-Myc is strongly linked to tumor formation. This study explores a safer alternative by removing c-Myc and compensating for its loss with targeted telomerase (TERT) gene therapy.
Targeted telomerase (TERT) gene therapy means carefully activating the enzyme telomerase only in specific cells, for limited periods, to help them maintain their ability to divide and function. Because telomerase can also enable cancer growth if overused, these therapies are designed to be selective and controlled, boosting cell renewal while minimizing the risk of uncontrolled cell proliferation (for more, see Jäger and Walter, 2016).
The biological problem the team aimed to solve lies in the trade-off between rejuvenation and safety. While c-Myc helps activate telomerase and maintain chromosome ends (telomeres), it also drives uncontrolled cell growth. Removing it makes reprogramming safer, but less effective at slowing aging. The researchers therefore combined a reduced set of Yamanaka factors Oct4, Sox2, and Klf4 (OSK) with direct activation of the TERT gene, which encodes the catalytic component of telomerase, the enzyme subunit that actively rebuilds telomeres during cell division. Telomerase is normally inactive in most adult cells, and its gradual loss contributes to cellular aging.
To test this strategy, the researchers used human lung fibroblast cells (MRC-5), a well-established model for studying human cellular aging. These cells naturally stop dividing after a fixed number of divisions, a phenomenon known as the Hayflick limit. The team introduced OSK alone, TERT alone, or both together into cells that had already reached an aged state. They then tracked changes in gene expression, cell viability, cell-cycle activity, and classic markers of cellular aging across multiple generations of cell division.
The results showed that the combined OSK + TERT treatment was more effective than either approach alone. Cells receiving both therapies expressed higher levels of genes associated with youth and regeneration, while showing reduced expression of aging and inflammatory genes. These cells also divided more actively, spent less time in growth-arrested states, and exhibited fewer biochemical markers of aging. Importantly, these benefits were achieved without reactivating high levels of c-Myc, suggesting a safer balance between rejuvenation and cancer risk in this in vitro system.
In sum, this study provides proof of concept that partial cellular reprogramming can be strengthened by pairing optimized Yamanaka factors with telomerase gene therapy. By separating the rejuvenating functions of c-Myc from its oncogenic (i.e., cancer-causing) risks, the researchers outline a potentially safer route toward anti-aging interventions. While the findings are currently limited to cultured human cells, they offer a clear framework for future animal studies and, eventually, therapies aimed at delaying age-related decline without triggering uncontrolled cell growth.

Are humans increasingly merging with technology? Image: Gerd Altmann, on Pixabay .
Taken together, these studies trace a clear progression: from enhancing cooperation between cells, to restoring immune coordination across organs, to carefully resetting cellular aging itself. In each case, technological success comes not from overriding biology, but from working within its constraints by revealing, stabilizing, or amplifying processes that already exist. The final study pushes this principle to its conceptual limit, not by addressing disease or aging directly, but by asking a deeper question about biological matter itself: can living molecules sustain the fragile states that underpin advanced technologies?
The developing capabilities of quantum computing and quantum systems could advance the processes. In a study published in August 2025 in the journal Nature, titled “A fluorescent-protein spin qubit”, researchers led by Jacob S. Feder, from the University of Chicago, report the creation of a new kind of quantum system that bridges biology and quantum physics. Specifically, they show that a fluorescent protein, similar to the green fluorescent protein (GFP) widely used in biology, can function as a spin qubit, the basic unit of information in many quantum technologies. A qubit, unlike a classical bit, can exist in a combination of states at once, making it powerful but also extremely sensitive to its environment. Demonstrating a qubit inside a biological-style molecule is a surprising and conceptually important step.
At the heart of the study is the discovery that the fluorescent protein’s internal electronic structure can support long-lived quantum spin states. When illuminated with light, the protein enters an excited state in which the spin of an electron can be precisely controlled and read out optically. In simple terms, the same property that makes the protein glow also allows researchers to “see” and manipulate its quantum state. This optical accessibility is crucial, because many solid-state qubits require complex hardware to control and measure them, whereas this protein-based qubit can be addressed using light alone.
The researchers carefully characterized how stable and controllable these spin states are. They showed that the protein’s spin qubit remains coherent. That is, it preserves its quantum information for surprisingly long times even at room temperature. This is notable because quantum systems are usually extremely fragile, losing coherence quickly unless they are kept at very low temperatures. The protein’s molecular structure appears to shield the spin from environmental noise, offering a kind of built-in protection that is rare in quantum materials.
Beyond demonstrating the qubit itself, the study highlights why this matters. Fluorescent proteins are already deeply embedded in biological research, used to label cells, track proteins, and study living systems. Embedding quantum functionality into such molecules opens the door to entirely new tools, such as quantum sensors that could operate inside cells or biological environments. In principle, these protein-based qubits could be used to measure tiny magnetic fields, temperature changes, or chemical environments with the tiniest quantum-level sensitivity in places where manufactured quantum devices cannot go.
In sum, this study shows that quantum information science does not have to be confined to ultra-clean environments. By demonstrating a functional spin qubit inside a fluorescent protein, the researchers reveal that biology-inspired molecules can host robust quantum states. This work not only expands the range of materials suitable for quantum technologies but also blurs the boundary between living systems and quantum devices.
From insects to humans, living bodies are built on intricate biological technologies refined by evolution long before modern engineering. Image: Gerd Altmann, on Pixabay .
Taken together, these studies suggest that the next era of technological progress in biology may be defined less by control than by fine-tuning. Whether enhancing mitochondrial sharing, restoring immune signaling, resetting cellular aging, or stabilizing quantum states inside biological molecules, each advance succeeds by working with the grain of living systems rather than against it. Biology, in this view, is not a fragile substrate to be overridden, but a highly organized medium whose internal logics can be amplified with care.
As science moves forward, the most powerful technologies may not be those that impose order from the outside, but those that recognize, and gently extend, the extraordinary capacities life has already evolved.
Want to learn more? Here are some TQR articles we think you’ll enjoy:
- Thinking in the Age of Machines: Global IQ Decline and the Rise of AI-Assisted Thinking
- Can Science Break Free from Paywalls? Technologies for Open Science Are Transforming Academic Publishing
- COP-30 in Belém: What Emerging Technologies Can and Can’t Deliver for Planetary Health
- The Science of the Paranormal: Could New Technologies Help Resolve Some of the Oldest Questions in Parapsychology?
- Digital Sovereignty: Cutting Dependence on Dominant Tech Companies
Have we made any errors? Please contact us at info@thequantumrecord.com so we can learn more and correct any unintended publication errors. Additionally, if you are an expert on this subject and would like to contribute to future content, please contact us. Our goal is to engage an ever-growing community of researchers to communicate—and reflect—on scientific and technological developments worldwide in plain language.