Image by Freekpik
By James Myers
It’s clear to see that there’s a revolution underway in transportation.
Despite their higher prices, battery-powered electric vehicles (EVs) are rapidly approaching, and in some cases have overtaken, sales of fossil fuel-powered cars and trucks.
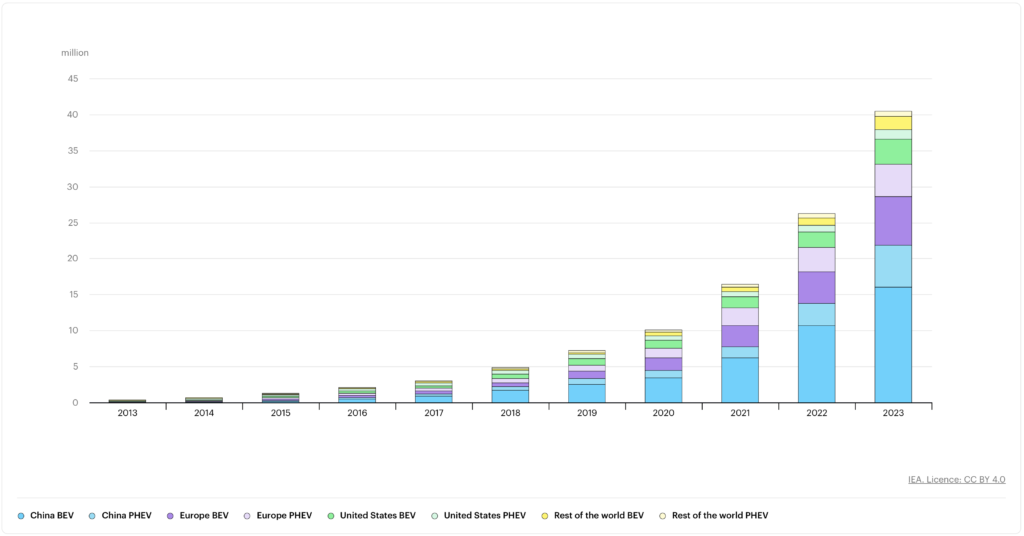
Global electric car stock, 2013-2023. Notes: BEV = battery electric vehicle; PHEV = plug-in hybrid vehicle. Includes passenger cars only. Image: Global EV Outlook 2024
In the US, where the gasoline-powered car was invented, EV’s now account for 18.7% of sales; in oil-rich Norway, EV’s predominate for 94% of new car buyers.
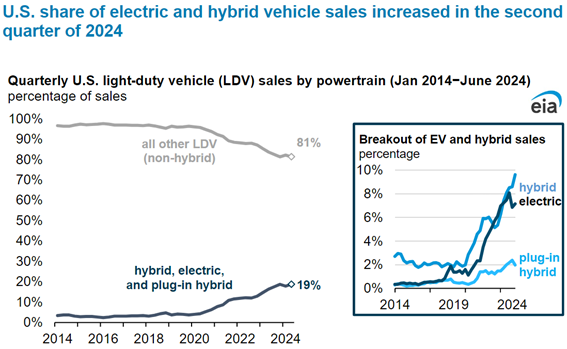
Combined U.S. sales of hybrid vehicles, plug-in hybrid electric vehicles, and battery electric vehicles (BEVs) represented 18.7% of total new light-duty vehicle (LDV) sales in 2Q24, according to estimates from Wards Intelligence. Image: US Energy Information Administration (EIA)
But are EV’s necessarily the way of the future?
Hydrogen-powered vehicles, although now scarce, are beginning to attract interest as a potentially more environmentally friendly option.
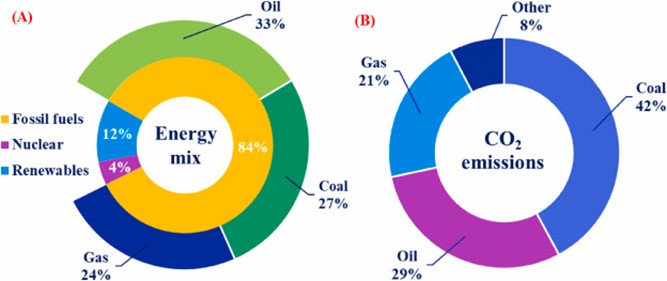
Global primary energy consumption and emissions of carbon dioxide (CO2), a greenhouse gas. Image from the January 2024 paper “Advancements in hydrogen production, storage, distribution and refuelling for a sustainable transport sector: Hydrogen fuel cell vehicles” published in the International Journal of Hydrogen Energy.
While EV’s don’t emit toxic pollution from burning fossil fuels, they come with significant environmental drawbacks.
Batteries are difficult and expensive to recycle at the end of their limited lives. They require the extraction of rare metals which places a major environmental toll on land and water and, according to Amnesty International, also leads to human rights abuses including children working in the mines. As well, the heavy weight of batteries decreases the lifespan of tires and increases the amount of airborne rubber particles.
Hydrogen fuel cells, by contrast, refill far more quickly, are lightweight, don’t require rare metal mining, don’t have limited lives, and emit only water and heat into the environment. However, producing, storing, and distributing hydrogen fuel is currently a technological and financial challenge.
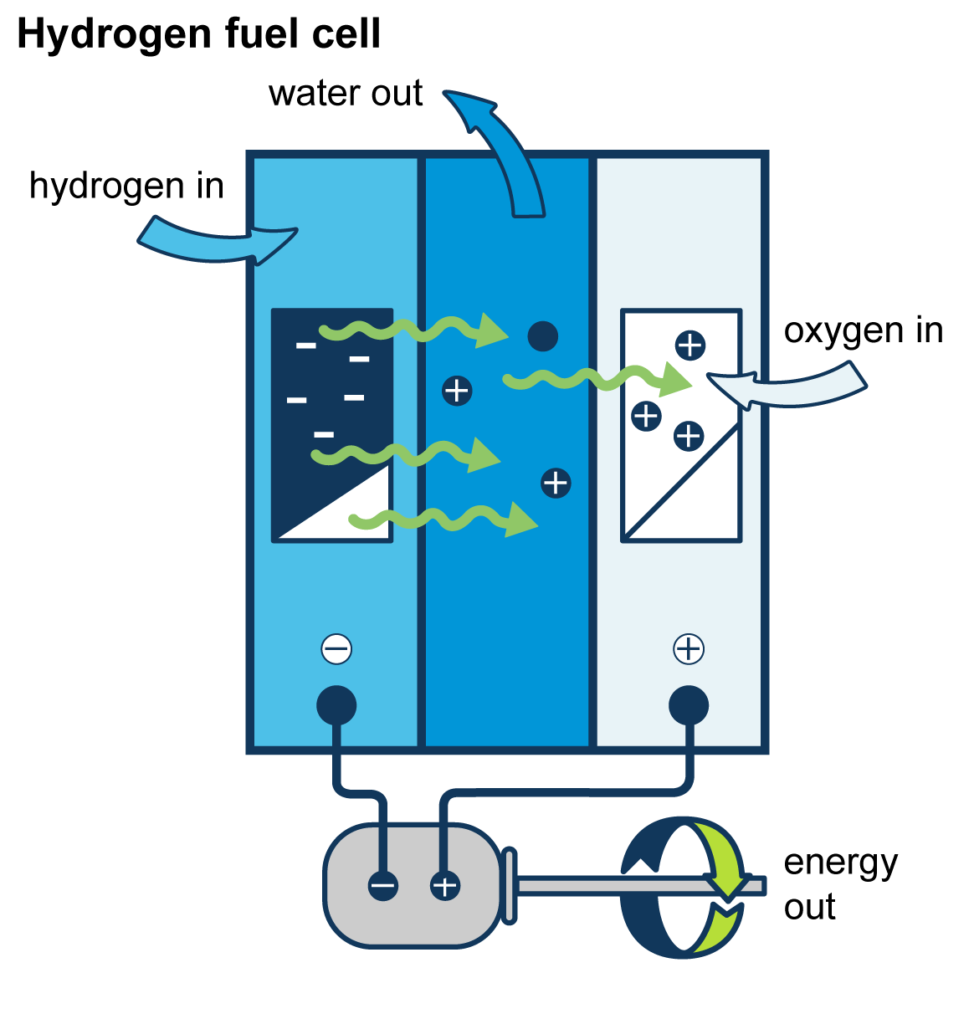
So what is a hydrogen fuel cell and how does it work?
In a fuel cell, a chemical reaction with an electrolyte separates hydrogen atoms into positively charged protons, which are in the atomic nucleus, and negatively charged electrons which orbit the nucleus. The atom-separating reaction with the electrolyte occurs in the middle of the fuel cell, between two electrical conductors with opposite charges, like in a regular battery. On one side of the fuel cell there’s a negative conductor called an anode and on the opposite side sits a positive conductor called a cathode.
When freed from the hydrogen nucleus, the protons create a positive charge in the electrolyte which the negatively charged electrons can’t penetrate.
As a result, while the protons can flow to the cathode because both are positively charged, the electrons are trapped at the anode where they are then drawn away in an electrical circuit connected to the motor. The circuit begins at the anode, runs through the motor, and after the electrons have performed their work, the circuit delivers them to the cathode. At the cathode, two electrons combine with two protons and oxygen from the air to form two water molecules (a water molecule, H2O, consists of two hydrogen atoms and one oxygen atom).
A hydrogen-powered vehicle contains a stack of many individual fuel cells producing electricity that charges batteries which drive an electric motor. The vehicle’s tailpipe emits only water and hot air.
The quest for efficient hydrogen fuel production is on.
Although hydrogen is the most abundant element in the universe, the financial and environmental costs of producing hydrogen fuel are significant barriers to the expansion of hydrogen-powered vehicles and fuelling stations.
In the United States, according to the US Energy Information Administration, nearly all hydrogen is consumed by industry for refining petroleum, treating metals, producing fertilizer and other chemicals, and processing foods. At present, hydrogen’s primary use for propulsion is in rocket fuel. The US currently has only about 60 hydrogen refuelling stations for vehicles, all of them in California because of the state’s Advanced Green Cars Program. In Canada, there are several hydrogen stations in British Columbia and one in Quebec City. In Europe, as of February 2024 there were 178 hydrogen fuelling stations, half of which were in Germany.
Honda, Hyundai, and Toyota are three companies that offer hydrogen vehicles, while other companies are developing the technology.
In the State of California, the beginning cost for a hydrogen-powered Honda CRV is currently $50,000. Hydrogen vehicles have a range of between 500 and 600 kilometres between fuelling, and while the price of hydrogen at the pump is considerably more than the cost of gas, a hydrogen-powered vehicle can travel about twice as far with an equivalent amount of fuel. Refuelling stations are similar to conventional gas stations, and filling a hydrogen vehicle requires only about 5 minutes compared to around 45 minutes to recharge an electric vehicle.
The most common method of producing hydrogen is to split methane (CH4, consisting of four hydrogen and one carbon atom) into carbon dioxide (CO2, consisting of two oxygen and one hydrogen atom) and two hydrogen atoms (H2). The carbon dioxide byproduct, however, is a greenhouse gas responsible for climate change and global warming, and for every kilogram of hydrogen fuel produced, approximately 10 kilograms of carbon dioxide are emitted into the atmosphere.
An environmentally sustainable method for producing hydrogen is called electrolysis, when a renewable energy source like solar is used to split water molecules (H2O) into their hydrogen and oxygen components. The problem with this greener option is its cost, currently about three times that of the carbon-intensive methane-splitting method, and therefore only about 1% of the world’s hydrogen is produced by electrolysis.
As the cost of capturing and converting solar energy continues to decrease, hydrogen production will become more economical.

Liquid-cooled fuel cell stack for use in light-, medium-, and heavy-duty vehicles. Image: Ballard Power
However, while solar energy might be plentiful and relatively inexpensive in an area like a desert, there are few areas where sources of both hydrogen and constant sunlight are found together. The costs of storing hydrogen and transporting it to refuelling stations are further barriers to economies of scale.
Researchers are turning their attention to increasing the efficiency of the catalysts used for the chemical reaction that separates protons and electrons in hydrogen atoms. If the density, or amount, of current that runs through the electrolyzer that sits between the anode and cathode in the fuel cell can be increased, the process becomes more efficient and less costly. Companies like Electric Hydrogen and ZeroAvia are investing in research to create more efficient fuel cells for ground vehicles and aircraft.
While technological constraints pose several problems to the efficient manufacturing of hydrogen fuel, there is an ongoing search for natural sources of ready-to-use hydrogen. According to the U.S. Gelogical Survey, it has long been known that hydrogen is produced by natural processes in the Earth’s geology, but “There’s just one problem: there’s little scientific information available about how much hydrogen is out there, or where it might be found.”
One such natural process, for example, is the reaction of groundwater with forsterite, a mineral containing olivine, in a process that produces the buildup of hydrogen in the surrounding rock.
Green in colour, olivine is a magnesium iron silicate and its rich iron content makes it a good catalyst for hydrogen production. Geologists know of dozens of other natural hydrogen-producing processes, and locating and tapping into reserves of natural hydrogen could avoid the significant costs and environmental consequences of manufactured hydrogen.

Image of forsterite, a natural mineral containing olivine, from the Smithsonian National Museum, downloaded from the USGS .
A paper published by the Royal Society of Chemistry in February, 2024 reports that there is a substantial rate of worldwide natural hydrogen production which is estimated only with a high degree of uncertainty; one such estimate places such annual production at 23 teragrams (Tg).
A teragram (Tg) is a unit of mass equal to one trillion grams (10¹² grams) or one million metric tons. The equivalent in imperial units is approximately 2.2 billion pounds. The teragram is often used in scientific contexts to quantify large-scale production or emissions.
The paper also reports that there are hundreds of known natural hydrogen sites, with the highest concentrations in Oman and Mali. The paper suggests two approaches to exploiting natural hydrogen, one being by “foraging” or exploring for high-concentration deposits, and the other being “farming,” involving the engineering of subsurface geological formations to promote the natural production of hydrogen.
What does the future hold for hydrogen?
The International Energy Agency notes that significant government funding is now available for hydrogen production through programs such as the US Hydrogen Production Tax Credit, the EU Important Projects of Common European Interest and the UK Low Carbon Hydrogen Business Model. As well, manufacturers of electrolysers, which trigger the chemical reaction in fuel cells, have ambitious plans to expand production by a factor of more than 100 times by 2030.
Expanded hydrogen availability would help to advance energy security, which is an increasing concern particularly in European countries like Germany and Italy needing to reduce their dependence on Russian gas after Russia’s invasion of Ukraine. As well, a greater awareness of the environmental damage caused by electric vehicle battery production and recycling is propelling interest in other forms of sustainable energy for transportation like hydrogen.
With the cost of a hydrogen-powered car already similar to that of a battery-powered vehicle, increased efficiencies in fuel cells and hydrogen production methods, as well as locating and engineering natural sources of hydrogen, could provide a major boost for the future of hydrogen power.
As the cost of producing, storing, and distributing the fuel decreases, the small number of hydrogen vehicles and fuelling stations now on the roads could increase significantly and provide an attractive and environmentally sustainable transportation option for consumers.
We would love to hear what you think of this article.
☞ Click here to complete our 2-minute Reader survey.
Craving more information? Check out these recommended TQR articles:
- Progress in Superconductivity Holds Promise for Clean Energy, Medicine, Quantum Computing, and More
- Advances in Nuclear Fusion Technology are Fueling the Dream of Limitless Clean Energy
- Clean Technologies and Technologies That Clean: Undoing the Climate Damage We Have Caused
- Harnessing Ocean Power: New Technologies Hold Promise for Clean and Cost-Effective Energy
- In Wiki Loves Earth Photos, Observers Share a Deep Knowledge of Nature’s Incredible Integration