The genome is the blueprint of a biological organism, and its code is now accessible to human manipulation.
The genetic code contains the instructions that enable individual cells to function properly, multiply, and form complex, viable organisms. However, errors and harmful mutations can occur in our genes that sometimes lead to diseases and disorders, and advances in the technology to edit them could lead to cures.
As our knowledge of genetics evolved, scientists began to consider the possibility of altering the genetic material of organisms. Over decades of research, various methods were developed to accomplish this feat. By far the fastest, cheapest, most accurate, and most efficient method comes from a system naturally used by bacteria to fight off viral infections. CRISPR technology uses the molecular machinery developed by bacteria to allow geneticists to edit specific parts of the genome by removing, adding, or changing sections of the DNA sequence.
CRISPR technology allows scientists to edit the DNA of living organisms.
With knowledge of how certain regions of DNA affect traits of organisms, such as genetic disorders or resistance to environmental stresses, scientists can use CRISPR to create improved DNA sequences in the lab. Among many possible applications, this has great potential in food production. For producers, edited plant genomes make it easier to create crops with higher yields and greater disease resistance. For consumers, we can make better products like gluten-free wheat or healthier oils. In fact, CRISPR-engineered foods are already a reality and are produced and consumed in many countries, even if many people are not yet aware of it.
However, this technology should be used with caution. Our knowledge of how DNA works as a whole and how different regions of the molecule interact and influence each other is still very limited. This means that we often do not know all the potential side effects of editing supposedly known regions of an organism’s DNA. In addition, we also need to consider the possibility of CRISPR-edited organisms breeding with native natural populations, creating mixed lineages that might affect the conservation of native species as well as the functioning of entire ecosystems.
The potential of CRISPR is not limited to plants or microorganisms.
It can be used to alter the DNA of any type of organism, including humans. At this point, the complexity of the ethical issues becomes immeasurable. On the one hand, CRISPR has the potential to transform medicine with the possibility of treating and preventing many diseases. On the other hand, editing a person’s genetic material with our incomplete knowledge of the potential collateral damage involves much greater risks than working with plants, for example. Also, the very thought of creating genetically “improved” humans immediately raises the danger that it could be used for eugenics, the discredited practice of more than a century ago. The complexity of this subject is one of the main reasons why research in this particular area of application has been slow.
“Eugenics is the scientifically erroneous and immoral theory of “racial improvement” and “planned breeding,” which gained popularity during the early 20th century. Eugenicists worldwide believed that they could perfect human beings and eliminate so-called social ills through genetics and heredity. They believed the use of methods such as involuntary sterilization, segregation and social exclusion would rid society of individuals deemed by them to be unfit.” [National Human Genome Research Institute.]
CRISPR is an acronym for “clustered regularly interspaced short palindromic repeats.”
This is both the name and description of a particular type of DNA segments discovered in 1987 by Dr. Yoshizumi Ishino at Osaka University. CRISPR came to Dr. Ishino’s attention by chance during an experiment he was conducting at the time. The organization of the repeats he discovered in the DNA of a bacterium caught his attention. Repeated DNA sequences are very common, but they are usually arranged sequentially, unlike this new type of repeats which were interspersed with other sequences. The acronym CRISPR was coined 14 years later by Dr. Francisco Mojica and Dr. Ruud Jansen, whose team laid the foundation for our understanding of these eccentric genetic constructs.
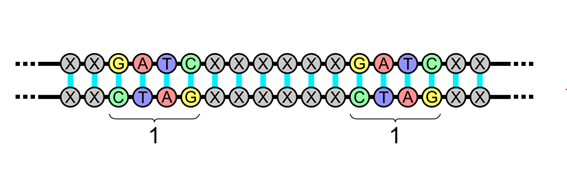
A palindromic sequence is a sequence in a double-stranded DNA or RNA molecule in which the reading in one strand is identical to the sequence in the same direction on the complementary strand.
In the early 2000s, scientists finally discovered that CRISPR plays a central role in the adaptive immunity of bacteria and archaea (a group of single-celled organisms that is generally very similar to bacteria) to viral infections. Viruses are obligate intracellular parasites, meaning that because they have no cells, they must use the machinery of other organisms’ cells to replicate. To this end, they may attempt to introduce their genetic material into cells to take control of their DNA replication mechanisms so that the cell begins to produce copies of the virus. To defend themselves against this type of attack, bacteria must be able to recognize and deactivate the genetic material of viruses when they enter their cell.
The CRISPR system in bacteria is similar to the adaptive immune system in humans.
When bacteria are infected with viruses, they not only try to destroy the virus but also assimilate parts of the viral DNA into their own genome. The bits of viral DNA that are added are stored in the bacterium’s DNA in segments by short palindromic repeats (see illustration), forming CRISPR arrays. When the bacterium is attacked again by the same virus (or a closely related one), it produces RNA segments based on these assimilated parts that find and bind to any foreign DNA with a matching sequence. Then the bacterium uses specific enzymes to cut the viral DNA and knock out the virus.
In 2020, Dr. Jennifer Doudna and Dr. Emmanuelle Charpentier received the Nobel Prize in Chemistry for being the first to successfully develop a DNA editing tool based on the CRISPR system. They studied the CRISPR system of the bacterium Streptococcus pyogenes and re-engineered it into a manageable system. This system uses an RNA sequence that, when combined with an enzyme that cuts DNA, can find and cut a specific target sequence in a cell’s DNA. Once the DNA is cut in the desired region, the scientists use the cell’s own repair system to add, replace or delete parts of the genetic material.

Dr. Emmanuelle Charpentier (left) and Dr. Jennifer Doudna (right) received the 2020 Nobel Prize in Chemistry for their contributions to the field of CRISPR research.
Since 2012, when Dr. Doudna and Dr. Charpentier published their breakthrough, many other systems using different enzymes have been developed and used in a variety of organisms, including yeast, fruit flies, plants, mosquitoes, fish, mice, monkeys, and humans. CRISPR technology has now advanced to the point where scientists can perform a wide range of different types of editing, from the less sophisticated removal of parts of DNA to the replacement of a single base of DNA code with another. There are even customized enzymes that, when used in the CRISPR system, do not change the DNA sequence at all, but instead activate or silence specific genes.
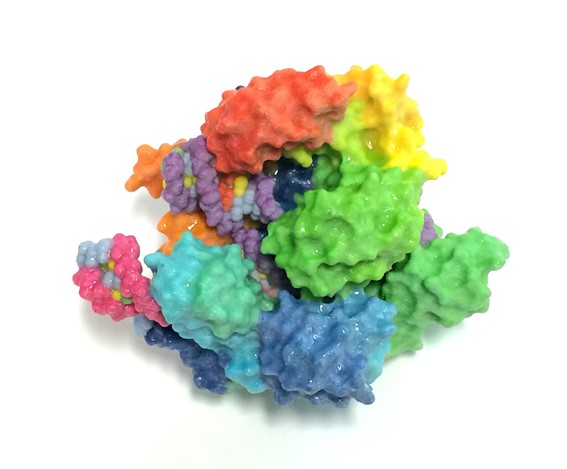
Representation of the 3D-structure of the Cas9 nuclease, the enzyme specialized for cutting DNA that is used in most CRISPR systems.
As technology advances and its potential applications become reality, we inevitably move toward gene editing in humans, and with it the ethical considerations become increasingly necessary.
Changes in the genome of somatic cells (i.e., cells in the body that are not sperm or eggs) remain confined to specific tissues and are not passed down through generations. This type of gene editing in humans could be and has been used, for example, to treat diseases. Because it remains confined to the individual that (hopefully) chose to undergo the procedure, this type of use for CRISPR involves comparatively less ethical concerns than the alternative.
The arguably greater ethical dilemma lies, however, in editing germline cells or the cells of embryos. The first most obvious problem with this type of application is that obtaining the person’s consent is not possible. Also, there are unpredictable effects that might arise from gene-editing that may only be evident later in the life of the person. When the DNA is cut for editing, there is space for random mutations to be introduced in the process of putting the DNA back together. These mutations may have negative effects on the organism. Plus, even if only the desired change in the DNA were to happen, we are very far from having a complete understanding of all the direct and indirect effects of editing any single human gene in the organism as a whole. To put it simply, the risks involved are inherently unpredictable.
We must also consider that, in the case of embryo genome editing, if some cells are not successfully edited, it may create mosaicism, in which the person will have a body composed of two different lineages of cells, one edited and one not edited. Lastly, since this type of genome editing is passed down through generations, the risks involved extend beyond the subjects of the experiment and carry the potential to propagate through the human genetic pool as they have children of their own.
An important landmark in the history of CRISPR research is the widely-condemned first experiment that created CRISPR-edited human babies, back in 2018. The research team, led by Dr. He Jiankui, used CRISPR to try to create human babies that were immune to HIV using in vitro fertilization, but the children did not have the desired resistance to the virus due to that type of mosaicism. For Dr. Jiankui, the controversy surrounding his experiments cost him his career and his freedom. For CRISPR research, that story appears to have delayed further experiments on the use of gene-editing technology to design humans.
In Vitro Fertilization: Unlike the simpler process of artificial insemination – in which sperm is placed in the uterus and conception happens otherwise normally – IVF involves combining eggs and sperm outside the body in a laboratory. Once an embryo or embryos form, they are placed in the uterus. [WebMD]
CRISPR has significant potential and is already being explored as a tool to treat or prevent various diseases, including cancer, heart disease, mental illness, HIV, hepatitis B, cystic fibrosis, hemophilia, and even high cholesterol.
To date, most research in these areas is still focused on animal models or isolated human cells, but this is a clear path to the day when CRISPR technology will be routinely used to treat human disease. While performing gene editing on human germline cells is currently illegal in most countries, with advances in research, we will certainly get to the point soon when at least specific, restricted uses will become legal.
With this in mind, it is important to spread information and awareness about CRISPR technology, its potential, and its complexity. Humans have unparalleled power to change the world, and CRISPR technology vastly expands that power to shape the very genomes that make us what we are. This may be the bottom line in the discussion of technology that allows us to engineer the humans of the next generations: What are the humans that we want to be?



