All cells in our bodies typically share the common goal of healthy function, and under normal conditions they know just what to do to achieve that collectively.
But sometimes, for reasons we do not yet fully understand, some cells change and begin to prioritize replication over cooperation. A trigger inside them causes them to replicate uncontrollably, converting them to cancer cells that drain massive resources from the body and form tumors. But what if we could override the behavior of cancer cells by providing them with a new set of instructions?
Cancer has long been one of the ultimate challenges to medicine, and many think this status is unlikely to change anytime soon.
Cancer is an umbrella term, not a single disease. In reality, there are more than 200 different types of cancer, and each has multiple subtypes that look and behave differently. There are a seemingly infinite number of mutations that can cause and arise from cancer. As a tumor grows, new mutations accumulate and create variation between the cells, so a single treatment may not affect all cells equally, and cells may even develop resistance. Finally, currently available treatments often involve significant suffering for patients and are extremely costly. For this reason, a cancer diagnosis in countries without public health care often leads to personal bankruptcy.
Yet, in the face of this baffling complexity, scientists around the world are working tirelessly to develop and improve cancer treatments.
Thanks to these efforts, the overall cancer death rate has steadily declined worldwide in recent decades. From 1990 to 2016, cancer deaths worldwide decreased by 17%. Governments and public health agencies also invest significantly in cancer research each year. The U.S. National Cancer Institute, for example, has invested tens of billions of dollars in cancer research over the past decade, and the investment has increased every year. More recently, a group of scientists at Cold Spring Harbor Laboratory, a private, nonprofit research institute, has come closer to understanding the mystery of what causes cells to become cancer cells.

Cold Spring Harbor Laboratory.
The research team led by Dr. David Spector, a researcher at Cold Spring Harbor Laboratory, has studied the inner workings of cancer cells in-depth, focusing on what enables them to migrate and invade different organs. The team believes that if they can figure out the molecular triggers that cause cells to stray from their natural cooperation and become cancer cells, molecular tools could be developed to put the cells back on track.
After nearly two decades of research, they may have found one of those molecular triggers. Now all the attention is on testing a molecular tool specifically designed to bypass the pattern of uncontrolled replication that that turns healthy cells into cancerous cells.
In recent decades, technological and therapeutic developments have vastly improved our ability to detect and remove or kill tumors in patients.
The tumor itself is just part of the problem. The biggest challenge for cancer patients is the ability of cancer cells to migrate to other tissues in the body and form new tumors in a process known as metastasis. Cancer cells often break away from their initial location, find their way into blood vessels, and travel through the bloodstream to other parts of the body. As the tumors then multiply in different tissues, it is almost impossible to eradicate them all.
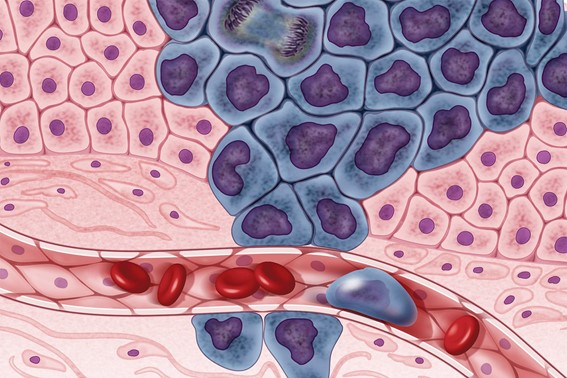
Illustration showing cancer cells (in purple) spreading to other parts of the body through the bloodstream.
Ribonucleic acid, or RNA, is an extremely versatile type of molecule, involved in almost every aspect and function of cells. RNA molecule roles include protein-coding and decoding, regulation of intracellular activities, and even gene expression. RNA received unprecedented media attention in 2020, the first year of the COVID -19 pandemic when the first mRNA vaccine was developed, highlighting the role of these molecules in instructing cells to produce certain proteins. RNA is so important that about 80% of the human genome is devoted to making different types of these molecules. Each type of RNA has a specific shape, size, and composition. They can combine in short or long chains or even circular molecules, depending on what best suits the task they perform in the cell.
But it is not always clear what a particular RNA molecule does. It is relatively easy to discern the role of RNA when it codes for or assembles proteins, but it may be difficult to assess the role of molecules involved in more subtle aspects of the cellular machinery, such as gene expression. These types of RNA are known as non-coding RNAs and make up a significant portion of the RNA molecules encoded by our genome.
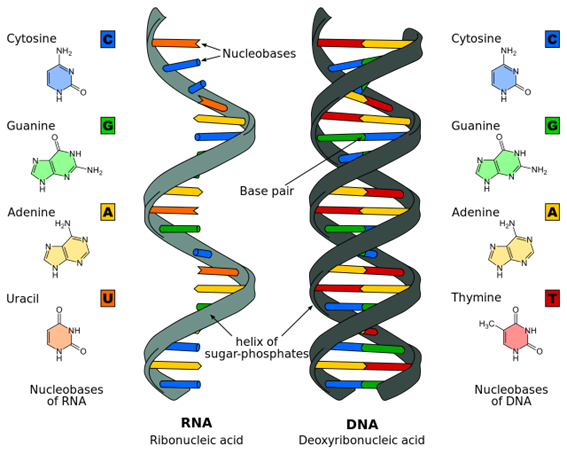
Illustration of the molecular structures of the RNA and DNA. The information in these molecules is encoded as specific sequences of nucleotides.
Out of over 60,000 genes that make up our genome, nearly 19,000 encode long (molecules with more than 200 nucleotides) non-coding RNA. Although we still do not know how many of these RNA molecules we produce in our cells, their sheer number is enough to inform us that they perform vital functions. One example is long non-coding RNAs that act as sponges by surrounding other molecules in cells to prevent their activity and then releasing them when they are needed. Another crucial function that long non-coding RNAs perform is gene expression, bringing proteins to specific genes to turn them on or off.
Dr. Spector focused his attention on the long non-coding RNA molecules that never leave the nucleus of the cell. He inferred that since they remain in the nucleus, they may be involved in the activation and deactivation of genes in DNA. The role of regulating gene expression is perhaps the most likely explanation for how cells can change their behavior so drastically when they become cancerous. Activation of the wrong genes at the wrong time can lead to malignant growth, as can deactivation of genes responsible for tumor suppression.
An 18-year search for the right molecule led the team to a long non-coding RNA called MALAT1. Their research showed that an intracellular excess of this particular type of RNA significantly changed the behavior of breast epithelial cells. When there is too much MALAT1 in breast epithelial cells, it is as if they forget what they are. Instead of exhibiting the normal healthy behaviors of the cells they are, such as supporting mammary gland function, they begin to behave like cancer cells whose only goal is to grow, multiply, and metastasize.
Once they identified their target, the new challenge was to develop a tool to fix the problem. Dr. Spector’s team hypothesized that if they could suppress the excess and restore the balance of MALAT1 in the cancer cells, the cells would remember their identity and return to their normal behavior. Working with researchers at Ionis Pharmaceuticals, they developed a molecule containing a sequence of nucleotides that is complementary to a specific region of the MALAT1. Because of this complementarity, this molecule can spontaneously bind to the target RNA. Once this happens, the cell understands that the RNA must be destroyed and sends a team of enzymes to degrade it.
The name of this molecule is MALAT1 RX, and the success shown by the first experimental results has excited the research team. Amazingly, after the amount of MALAT1 in the cells drops to a healthy level, the cells regain some of their biological identity – they remember what they are and what they are supposed to do. So far, the MALAT1 RX has been able to stop cancer progression in mice and also in tumors grown in the lab from samples from human cancer patients.
The team is now preparing for the next big step: experimenting with the treatment in live human patients in a clinical trial. Discussions with the FDA have already begun, and Dr. Spector hopes they can begin human trials in a year or two. The potential for this new technology to treat cancer and a range of other diseases by restoring cellular memory is both promising and very significant. The world will be hoping for the success of this RNA treatment, and it is a story we will be following closely as it reaches trial phase.



