
Image generated using Ideogram AI.
By Mariana Meneses
The first successful In vitro fertilization (IVF) pregnancy and live birth occurred in 1978.
Dr. Patrick Steptoe and Dr. Robert Edwards performed the procedure, fertilizing egg with sperm in a test tube, in England. It resulted in the birth of Louise Brown on July 25, 1978, who is now a global IVF advocate and speaker. This was a groundbreaking process that marked a significant milestone in the history of reproductive medicine and gave hope to countless individuals and couples struggling with infertility.
As fertility treatments have become widely used, new technology is advancing effectiveness and options.
According to the U.S. Society of Assisted Reproductive Technology (SART), over 1 million babies were born in the United States between 1987 and 2015 with the assistance of fertility treatments such as IVF or other related technologies. The average cost of a single IVF cycle in the U.S. is between $10,000 and $15,000, and is dependent upon insurance coverage, patient characteristics, and the treatment center.
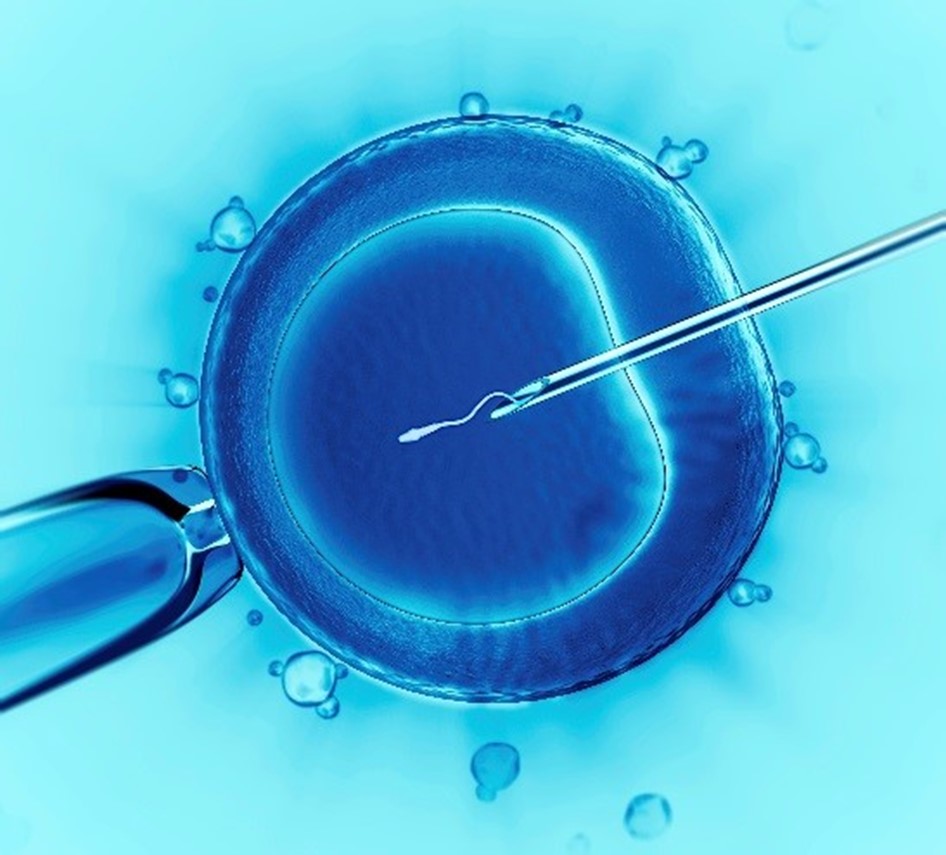
Illustration of in vitro (“in glass”) fertilization. Credit: US Government.
IVF is a multifaceted series of medical procedures aimed at aiding fertility, addressing genetic concerns, and facilitating conception.
Within the IVF process, mature eggs are retrieved from the ovaries and then fertilized with sperm in a controlled laboratory setting. Once fertilization occurs, the resulting embryos are subsequently transferred into the uterus. This comprehensive cycle of IVF typically spans around three weeks.
Known for its effectiveness, IVF stands out as a potent assisted reproductive technology. It can involve the utilization of a couple’s own eggs and sperm or incorporate reproductive materials from donors, either known or anonymously. In some instances, a woman acts as a gestational carrier, which is the preferred term for what is commonly referred to as a surrogate, who carries and nurtures the implanted embryo and becomes part of the process.
The potential for achieving a successful, healthy pregnancy through IVF hinges on various factors, including the woman’s age and the underlying causes of infertility. However, it’s important to note that IVF demands a considerable investment of time, money, and may entail invasive procedures. Moreover, in cases where multiple embryos are implanted in the uterus, IVF can lead to pregnancies with more than one fetus.
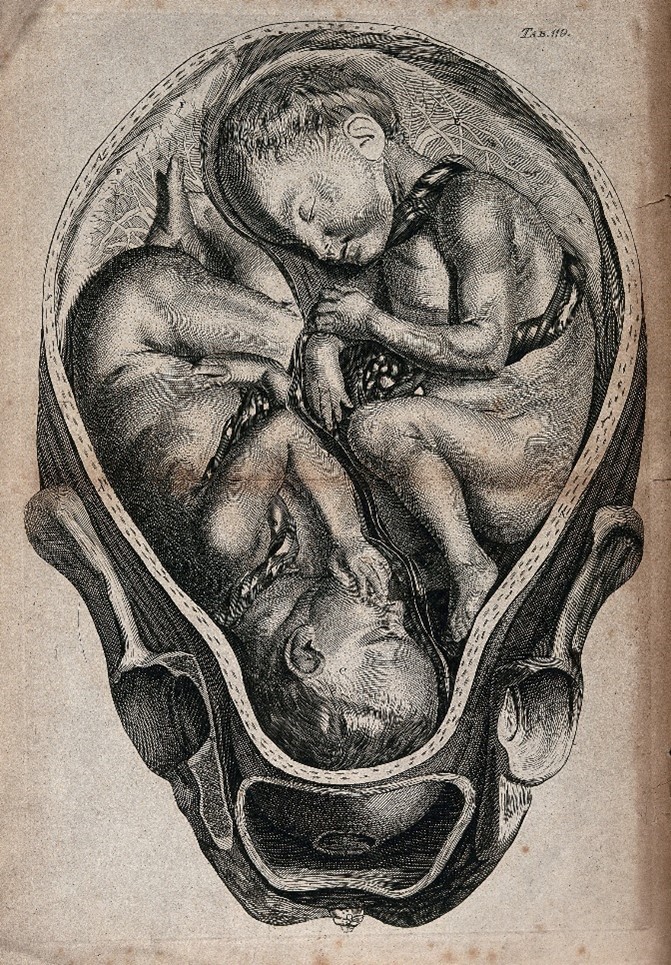
IVF can lead to multiple pregnancies. Credit: J. van Rymsdyck.
A new AI technology from AIVF, an Israeli company, helps fertility doctors pick the most viable embryos for IVF, tackling low success rates and high demand.
An increasing number of women are opting to freeze their eggs as a means to postpone childbirth and concentrate on advancing their careers. This trend has led to a surge in demand for in vitro fertilization services. As women prioritize professional development and personal aspirations, the desire to preserve fertility through egg freezing has become a pivotal aspect of family planning, consequently driving the need for IVF procedures to realize their reproductive goals later in life.
A report by BBC Worklife shows that, during the pandemic, many fertility clinics saw a huge jump in interest for egg freezing procedures. It suggests that in the US, egg freezing retrievals increased by 39% from pre-pandemic levels, while in the UK enquiries rose by as much as 50% in the summer of 2020 compared to the prior year.
The new technology is an AI-powered embryo evaluation software called EMA.
The software is designed to process vast amounts of data, beyond what the human eye can detect, to simplify the embryo selection process. Daniella Gilboa, an embryologist who is co-founder and CEO of AIVF, told Fox News that IVF’s success rates are about 23% to 25%, which means about only one in four result in pregnancy.
One of the biggest challenges is that IVF clinics can’t keep up with the growing demand. In the U.S., only 20% of the need is served, which means 80% of women who require the procedure are giving up on the dream of having a child.
Image generated using Ideogram AI.
AI is helping not only to create babies in the lab, but also human organoids, which are organ-like tissues grown in the laboratory.
According to Sci Tech Daily, in August 2023, Japanese scientists at RIKEN institute developed a cube-like hydrogel device that makes it much easier to create 3D organ-like structures in the lab. These structures can help us understand how genes work, and it could change how we test medicines and grow artificial organs. The team of scientists, led by Masaya Hagiwara, also demonstrated the ability to use the device to build organoids that faithfully reproduce the asymmetry, or differences, in gene expression that characterizes the actual development of organisms.
Creating organoids that function like real tissues is vital for developing medicines, since it is necessary to understand how drugs move through various tissues.
Organoids also help to provide insights into the process of organ development itself and are a stepping stone on the way to growing whole organs for transplantation in patients. However, creating life-like organoids has proven difficult since, in nature, tissues develop through an elaborate dance that involves chemical gradients and physical scaffolds that guide cells into certain 3D patterns. In contrast, lab-grown organoids typically develop in homogeneous conditions—creating simple balls of similar cells—or by using technologies which require both very sophisticated equipment and skills.
It is worth noticing that creating organoids, which are tiny, life-like structures made from stem cells, raises several ethical questions.
These include obtaining permission from donors, the selling of the structures, their use in personalized medicine and transplants, the possibility of their developing consciousness, their use in creating part-human, part-animal organisms, and their use in creating gastruloids, which are tiny structures that resemble early embryos and are used to study how mammals, including humans, develop.
The innovative technique allows scientists to control the environment around groups of cells, using less costly and specialized equipment. The method involves confining layers of hydrogels—substances made up mostly of water—with different physical and chemical properties inside a cube-shaped culture vessel. And cells could be inserted in ways to allow the creation of a range of tissue types.
Thus, AI is playing an increasingly important role in the field of human reproduction and organ transplantation.
AI-powered embryo evaluation software will increasingly assist fertility doctors select the best embryos for IVF, and AI-powered techniques are being developed to create more life-like organoids in the lab. These advances have the potential to improve the success rates of IVF, make organ transplantation more accessible, and help us better understand the development of human bodies.
Craving intellectual stimulation? Don’t miss these captivating TQR articles:
- What If We Could Cure Cancer by Telling Cancer Cells to Get Better?
- Reversing the Ageing Process? New Discovery Points to The Body’s Relationship With Time
- New Understanding of How Cells Communicate May Supercharge Medical Advances
- Discovering the Human Code: the Most Complete Human Genome Ever Created
- CRISPR Technology: Editing the Genetic Code, From Plants to Humans



